Research Overview
 A central theme of our research program is epigenetic control of cell proliferation and differentiation with emphasis on mammalian development and compromised regulation that is linked to disease. We pioneered the characterization of transcriptional regulation that mediates cell cycle control utilizing multi-omic and spatial transcriptomic strategies. Currently, the laboratory is investigating gene regulatory mechanisms that control competency for cell cycle progression at the G1/S phase transition in normal and tumor cells, as well as in cancer stem cells, and human embryonic stem cells that have a characteristic abbreviated pluripotent cell cycle.
A central theme of our research program is epigenetic control of cell proliferation and differentiation with emphasis on mammalian development and compromised regulation that is linked to disease. We pioneered the characterization of transcriptional regulation that mediates cell cycle control utilizing multi-omic and spatial transcriptomic strategies. Currently, the laboratory is investigating gene regulatory mechanisms that control competency for cell cycle progression at the G1/S phase transition in normal and tumor cells, as well as in cancer stem cells, and human embryonic stem cells that have a characteristic abbreviated pluripotent cell cycle.
A second field in which our laboratory is making a major impact is skeletal biology, where we established the foundation for addressing bone tissue-specific gene expression. Current initiatives include microRNA/long-non-coding RNA mediated control and molecular, cellular, biochemical, and in vivo genetic parameters of skeletal development and bone remodeling, aberrations that accompany the onset and progression of skeletal disease, as well as perturbations related to breast and prostate cancer metastasis to bone.
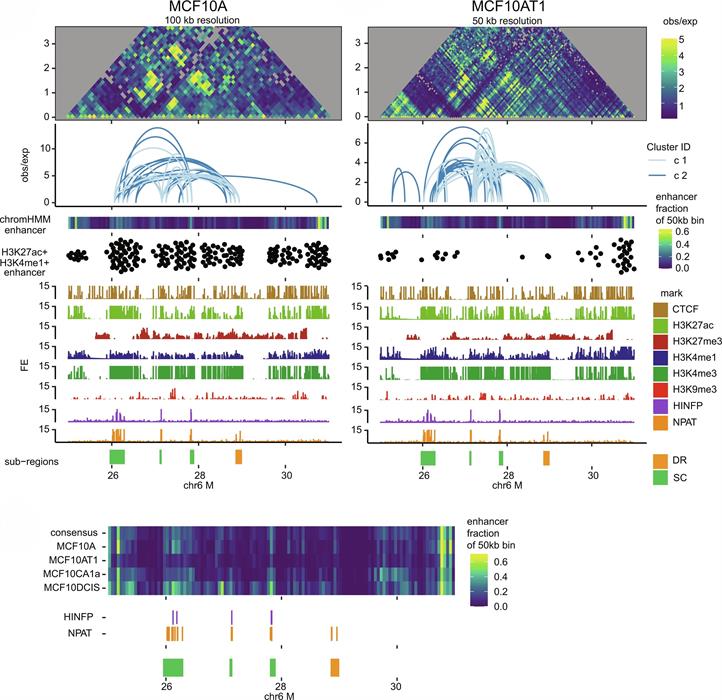
Our research group has been instrumental in defining functional relationships between the subnuclear organization of regulatory proteins, higher-order chromatin organization and gene expression. We have made important contributions to mechanisms that mediate combinatorial organization and assembly of regulatory machinery for gene expression in nuclear microenvironments. Currently, we are examining how nuclear architecture supports epigenetic control of cell fate and lineage commitment in biological control and cancer, mitotic gene bookmarking and oncofetal epigenetic control are being addressed. The architectural parameters of regulatory networks that are obligatory for the fidelity of gene expression are being clarified as a basis for therapeutic strategies with high specificity and reduced off-target effects.