Research
Mechanisms regulating factor V endocytosis by megakaryocytes
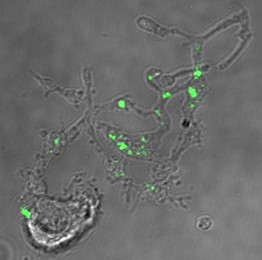 Factor Va, is a critical non-enzymatic, protein cofactor in blood clot formation via the formation of thrombin by the prothrombinase complex assembled on the surface of activated platelets adhered at sites of vascular injury. The procofactor, factor V, exists in two whole blood pools: 75-80% circulates in plasma (liquid portion of blood) while the remainder is stored in alpha-granules of blood cells called platelets from where it is released upon blood vessel injury and platelet activation. While both plasma and platelet factor Va function efficiently in blood clotting, the platelet molecule forms the more physiologically relevant cofactor pool in the normal blood clotting response as its local concentration at sites of blood vessel injury is >100-fold higher than plasma factor The entire platelet-derived factor Va pool originates via endocytosis of the procofactor, factor V, from plasma by megakaryocytes, the platelet precursor cells. Factor V endocytosis by megakaryocytes is specific, clathrin-dependent, and mediated by a two receptor system. Factor V initially binds to a specific factor V receptor expressed only on megakaryocytes able to endocytose factor V. This binding event facilitates an interaction between a second factor V molecule and LDL receptor-related protein-1 (LRP-1), an endocytic receptor, which subsequently mediates the endocytosis of bound factor V. These combined observations represent a unique role for LRP-1 in endocytosis of a coagulation protein not destined for lysosomal degradation. Rather, subsequent to its endocytosis, factor V is functionally modified, trafficked to, and stored in alpha-granules. The overall goal of these studies is to further define how plasma-derived factor V is acquired by megakaryocytes using megakaryocyte derived ex vivo from human bone marrow or umbilical cord blood, and how its intracellular transport and modifications yield a physically and functionally distinct platelet protein. Delineation of the cellular processes regulating platelet acquisition of this unique factor V/Va pool is essential for understanding how fibrin formation is regulated at the activated platelet surface.
Factor Va, is a critical non-enzymatic, protein cofactor in blood clot formation via the formation of thrombin by the prothrombinase complex assembled on the surface of activated platelets adhered at sites of vascular injury. The procofactor, factor V, exists in two whole blood pools: 75-80% circulates in plasma (liquid portion of blood) while the remainder is stored in alpha-granules of blood cells called platelets from where it is released upon blood vessel injury and platelet activation. While both plasma and platelet factor Va function efficiently in blood clotting, the platelet molecule forms the more physiologically relevant cofactor pool in the normal blood clotting response as its local concentration at sites of blood vessel injury is >100-fold higher than plasma factor The entire platelet-derived factor Va pool originates via endocytosis of the procofactor, factor V, from plasma by megakaryocytes, the platelet precursor cells. Factor V endocytosis by megakaryocytes is specific, clathrin-dependent, and mediated by a two receptor system. Factor V initially binds to a specific factor V receptor expressed only on megakaryocytes able to endocytose factor V. This binding event facilitates an interaction between a second factor V molecule and LDL receptor-related protein-1 (LRP-1), an endocytic receptor, which subsequently mediates the endocytosis of bound factor V. These combined observations represent a unique role for LRP-1 in endocytosis of a coagulation protein not destined for lysosomal degradation. Rather, subsequent to its endocytosis, factor V is functionally modified, trafficked to, and stored in alpha-granules. The overall goal of these studies is to further define how plasma-derived factor V is acquired by megakaryocytes using megakaryocyte derived ex vivo from human bone marrow or umbilical cord blood, and how its intracellular transport and modifications yield a physically and functionally distinct platelet protein. Delineation of the cellular processes regulating platelet acquisition of this unique factor V/Va pool is essential for understanding how fibrin formation is regulated at the activated platelet surface.
Characterization of specific nanobodies from a yeast surface display library
In another research project being performed in collaboration with Dr. Russell Tracy, nanobodies produced recombinantly in yeast are being characterized. Nanobodies are the antigen recognition sites of naturally-occurring camelid single domain antibodies. Nanobodies that recognize the receptor binding domain of the SARS–CoV-2 spike protein and factor V have been identified and are being characterized using standard cellular and molecular techniques. In another study, nanobodies against a marker of inflammation, soluble CD14, are being identified.